Our Research
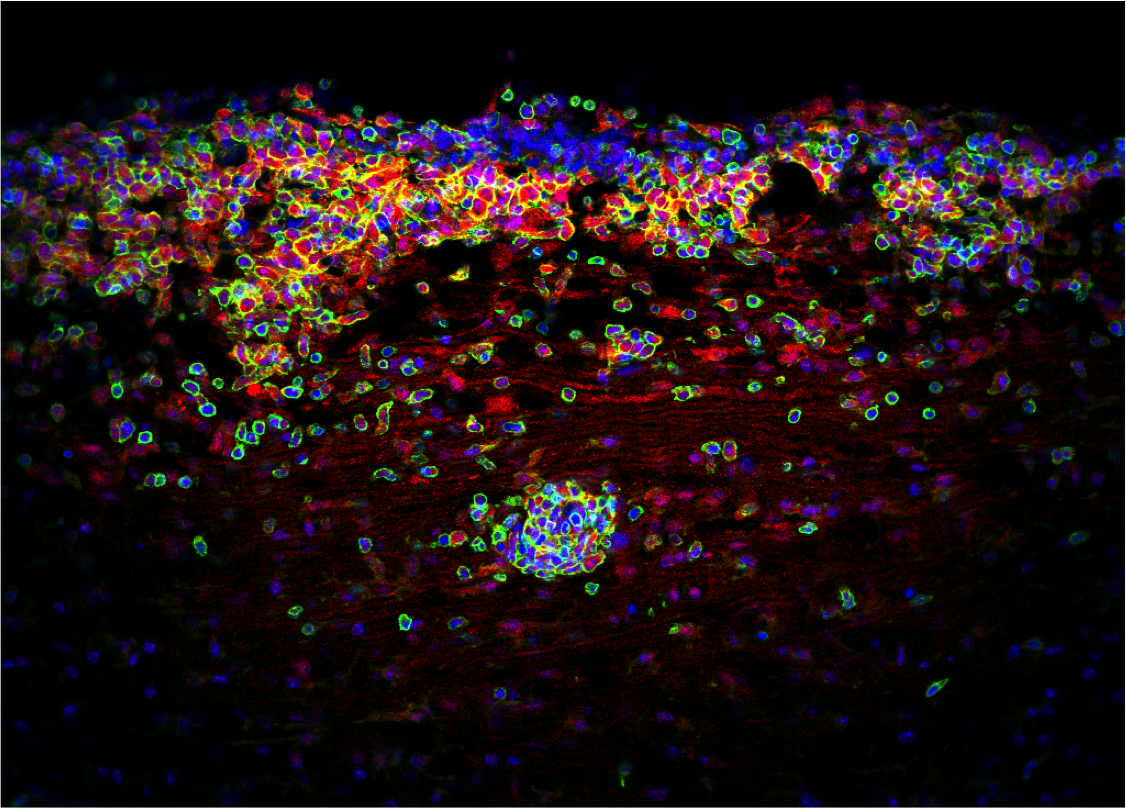
Cellular Senescence and Neuroinflammation
Cellular senescence, a state where cells stop dividing and release inflammatory molecules, plays a significant role in brain aging and disease. Our research explores how these senescent cells contribute to chronic neuroinflammation, impacting myelination and exacerbating conditions like multiple sclerosis. We aim to understand these mechanisms to identify new therapeutic targets.
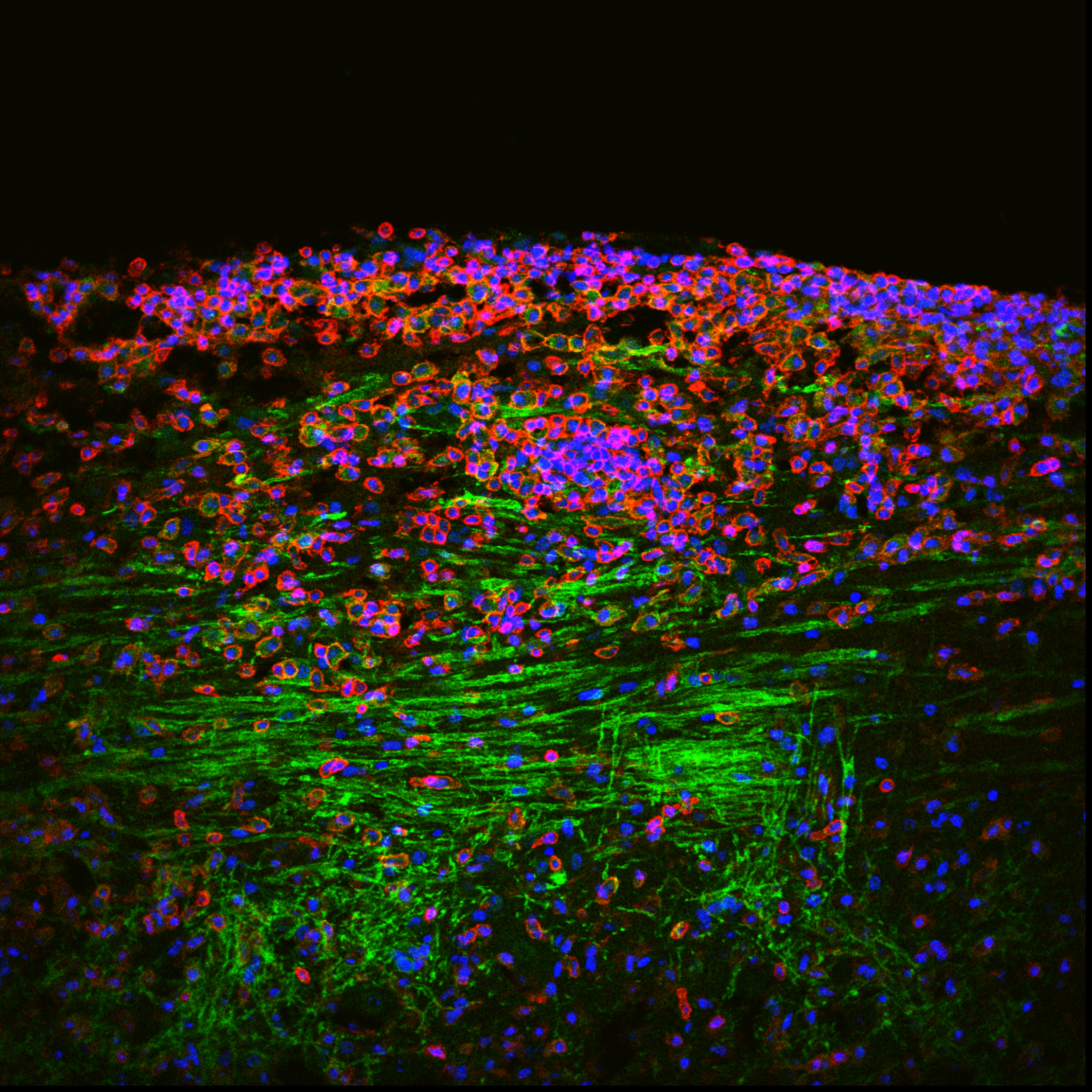
Senescent Oligodendrocyte Precursor Cells (OPCs) and Remyelination Failure
OPCs are vital for repairing myelin, the protective sheath around nerve fibers. In diseases like multiple sclerosis and with advancing age, OPCs can enter a senescent state, impairing their ability to differentiate and regenerate myelin. Our research focuses on understanding how OPC senescence contributes to remyelination failure, with the goal of developing strategies to rejuvenate these cells and promote myelin repair.

Telomere Shortening in OPCs and Impaired Myelin Repair
Telomeres, the protective caps at the ends of our chromosomes, shorten with each cell division, contributing to cellular aging. Our lab investigates how this telomere shortening in OPCs impairs their differentiation capacity. We aim to understand if this process is a key driver of remyelination failure in aging and demyelinating conditions, potentially identifying new strategies to enhance myelin repair by targeting telomere biology.
Extracellular Matrix (ECM) Changes: Impact on Brain Aging and Myelin Repair
The ECM is the vital scaffold surrounding brain cells, influencing their behavior and function. With age, the ECM undergoes significant alterations in its composition and structure. These changes can create an environment that hinders the ability of OPCs to differentiate and effectively repair myelin. Our research investigates how these dynamic ECM modifications contribute to remyelination failure, aiming to identify strategies to restore a more supportive, pro-regenerative environment in the aging and diseased brain.